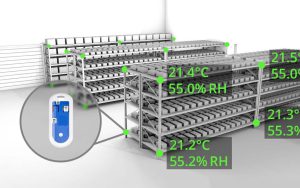
What is thermal validation in the pharmaceutical industry?
Thermal validation in the pharmaceutical industry is a crucial process that involves the systematic assessment and confirmation of temperature-related parameters within various components, equipment, and processes. This validation ensures that the thermal conditions in pharmaceutical manufacturing and storage environments comply with regulatory standards, guaranteeing product quality and safety.
What is validation testing in the pharma industry?
Validation testing in the pharmaceutical industry is a systematic and documented process of confirming that a system, process, or equipment consistently produces results that meet predetermined specifications. It encompasses various aspects, including thermal validation, to ensure the reliability, accuracy, and compliance of pharmaceutical processes.
What are thermal validation guidelines?
Thermal validation guidelines provide a framework for establishing and maintaining the thermal control of critical processes in the pharmaceutical industry. These guidelines typically include standards set by regulatory authorities, such as the FDA and WHO, and outline the procedures and criteria for conducting thermal validation to meet quality and safety requirements.
What is area validation in pharma?
Area validation in the pharmaceutical industry involves assessing and validating the environmental conditions within specific areas of a facility, such as manufacturing, storage, or cleanrooms. This ensures that the controlled environments maintain the required temperature parameters to guarantee the quality of pharmaceutical products.
Why do we need thermal validation in pharma?
Thermal validation in the pharmaceutical industry is essential to ensure that processes and equipment operate within specified temperature ranges. This is critical for maintaining the stability and efficacy of pharmaceutical products, preventing degradation, and complying with regulatory requirements for quality assurance and patient safety.
Why is validation important in pharmaceutical manufacturing?
Validation is crucial in pharmaceutical manufacturing as it ensures that processes, systems, and equipment consistently meet predetermined specifications. This quality assurance practice is vital for preventing defects, ensuring product safety, and complying with regulatory standards, ultimately guaranteeing the integrity and efficacy of pharmaceutical products.
Which thermal validation equipment is used in the pharma industry?
Common thermal validation equipment used in the pharmaceutical industry includes data loggers, temperature sensors, thermal mapping tools, and specialized monitoring systems. These instruments play a key role in assessing and validating temperature control within various pharmaceutical processes and environments.
What are the steps in thermal validation?
The steps in thermal validation typically include planning, protocol development, execution of tests, data analysis, and documentation. The process involves defining critical parameters, conducting thermal mapping, and assessing the performance of equipment or processes under different temperature conditions to ensure compliance with regulatory requirements.
What is temperature test validation?
Temperature test validation involves evaluating and confirming that specific equipment or processes can consistently achieve and maintain predetermined temperature parameters. This testing is crucial in the pharmaceutical industry to ensure the reliability of temperature-sensitive processes and comply with regulatory standards.
What is temperature mapping validation?
Temperature mapping validation involves systematically assessing and documenting temperature variations within a defined space, such as storage areas or transportation containers. This process ensures uniform temperature distribution, identifies potential hot or cold spots, and verifies compliance with temperature control requirements in the pharmaceutical industry.
How can your Thermal Validation services directly impact my bottom line?
Beyond just meeting regulations, our Thermal Validation services deliver tangible benefits to your bottom line:
Reduced Product Loss: Minimize temperature-related product degradation, saving costs associated with recalls and wasted batches.
Improved Process Efficiency: Identify and eliminate thermal deviations, optimize production, and reduce operating costs.
Enhanced Supply Chain Integrity: Validate temperature control during transportation and storage, ensuring product potency and shelf life, and reducing supply chain disruptions and waste.
Data-driven Risk Management: Gain valuable insights into thermal risks and vulnerabilities, empowering proactive improvement decisions that save resources and prevent costly issues.
What types of Thermal Validation services do you offer?
Equipment Qualification (EQ): Verifies equipment and systems function within defined temperature parameters.
Process Qualification (PQ): Evaluates how well the process maintains required temperatures during simulated or actual production.
Mapping and Monitoring: Creates detailed temperature maps of controlled environments and implements continuous monitoring systems to detect deviations.
Transportation Validation: Tests and confirms packaging and shipping processes to maintain product temperature integrity.
How can I ensure my Thermal Validation plan aligns with regulatory requirements?
We have extensive experience adhering to FDA, EMA, and other relevant regulations.
We guide you through the validation process, ensuring your protocols and documentation meet all regulatory standards.
What makes your Thermal Validation services stand out?
Experienced and specialized team: Our team boasts in-depth knowledge of pharmaceutical processes and thermal validation best practices.
Customized approach: We tailor our services to your specific needs and equipment, ensuring a comprehensive and effective validation process.
Advanced technology and tools: We utilize cutting-edge data acquisition and analysis tools for accurate and efficient validation.
Clear communication and collaboration: We keep you informed throughout the process and address any concerns promptly.