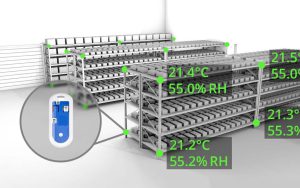
Was your cleanroom designed and constructed in accordance with ISO 14644 standards?
Yes, our cleanroom was meticulously designed and constructed in strict accordance with ISO 14644 standards, ensuring that it meets the specified cleanliness and environmental control requirements for its intended use.
What type of HVAC system does your cleanroom use?
Our cleanroom is equipped with a state-of-the-art HVAC system designed to maintain precise control over temperature, humidity, and particulate levels. The HVAC system is tailored to the cleanroom’s classification and operational needs, aligning with industry standards.
What records are maintained of cleanroom monitoring data?
We maintain comprehensive records of cleanroom monitoring data, including particulate counts, temperature, humidity, and pressure differentials. These records are critical for demonstrating compliance with cleanliness standards and ensuring the controlled environment’s integrity.
What documentation is required for cleanroom validation?
Cleanroom validation documentation includes detailed protocols, reports, and records related to design, installation, operation, and performance qualification. This documentation is essential for demonstrating that the cleanroom consistently meets specified standards and requirements.
Who is responsible for maintaining cleanroom validation documentation?
The responsibility for maintaining cleanroom validation documentation typically lies with a designated quality assurance or validation team within our organization. These professionals ensure that all documentation is up-to-date, accurate, and readily accessible for audits or reviews.
How is cleanroom validation documentation reviewed and updated?
Cleanroom validation documentation undergoes regular reviews and updates as part of our quality management processes. Any changes in equipment, processes, or standards prompt a thorough review and necessary updates to ensure ongoing compliance and accuracy.
Where is cleanroom validation documentation stored?
Cleanroom validation documentation is securely stored in a controlled and accessible electronic or physical repository within our organization. This ensures that the documentation is well-organized, easily retrievable, and protected against loss or unauthorized access.
How is cleanroom validation documentation accessed?
Access to cleanroom validation documentation is typically restricted to authorized personnel, such as members of the quality assurance or validation team. Strict access controls and permissions are in place to maintain the confidentiality and integrity of the documentation.
How often is your cleanroom audited?
Our cleanroom undergoes regular audits as part of our compliance and quality assurance program. The frequency of audits is determined based on risk assessments, regulatory requirements, and internal quality standards.
Who conducts cleanroom audits?
Cleanroom audits are conducted by qualified internal or external auditors with expertise in cleanroom validation and compliance. These individuals may be part of the organization’s quality assurance team or may be independent third-party auditors. Choose Incepbio for your Cleanroom Validation Services.
What is the scope of cleanroom audits?
Cleanroom audits encompass a thorough examination of all aspects related to cleanliness, environmental controls, and compliance with validation documentation. The scope includes assessing equipment, processes, monitoring practices, and the overall adherence to regulatory and industry standards.
How can your Cleanroom Validation services help me prevent product recalls and ensure compliance?
Our comprehensive protocols and rigorous testing procedures identify and address potential contamination risks before they impact your production.
We stay updated on the latest regulatory requirements and ensure your cleanrooms meet all FDA, EMA, and other relevant standards, minimizing the risk of recalls and fines.
I already have internal validation procedures. Why outsource your service?
Our team of experienced professionals brings specialized expertise and knowledge of best practices. We offer an objective assessment of your cleanroom performance, identifying potential blind spots and recommending improvements that might be missed internally.
Additionally, outsourcing validation saves you time and resources, allowing your team to focus on core production activities.
Can you customize your Cleanroom Validation services to my specific needs and cleanroom configuration?
Absolutely! We understand that every cleanroom is unique, and we tailor our approach to fit your specific processes and equipment.
We work closely with you to understand your requirements and develop a customized validation plan that addresses your concerns and risk factors.
How frequently should I conduct Cleanroom Validation?
The frequency depends on your cleanroom classification, regulatory requirements, and production processes.
We recommend a risk-based approach, with more frequent validations for critical processes and environments.
We can help you define the optimal validation schedule based on your specific needs.
What type of reports and documentation do I receive after the validation process?
We provide detailed reports that document the validation results, identify any findings or deviations, and recommend corrective actions if necessary.
These reports serve as valuable documentation for regulatory compliance and continuous process improvement.